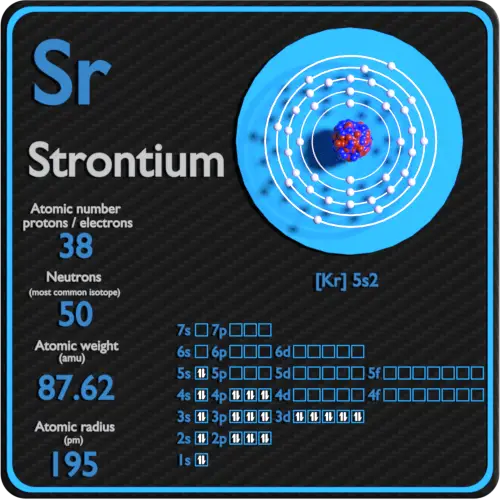
What Element Has 38 Protons and 50 Neutrons
There are 38 positively charged particles in its nucleus. 1 atomic mass unit is defined as 112 of the mass of a single carbon-12 atom.
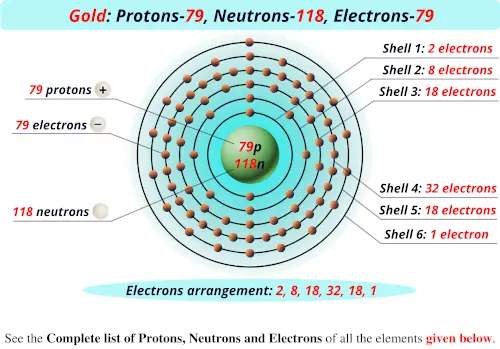
Protons Neutrons Electrons Of All Elements List Images
Additionally what atom has 28 protons and 32 neutrons.
. The nucleus of phosphorus-32 contains 15 protons and 17 neutrons one more neutron than the most common isotope of phosphorus phosphorus-31. There are 38 positively charged particles in its nucleus. 112 rows 38.
Write the nuclear symbol for the element. For strontium Z 38. Which element has 32 protons in its nucleus.
Likewise which elements are isotopes. Similarly what element has 64 neutrons 48 electrons. 32 an isotope of this element.
Strontium has 38 protons necessarily and 38 electrons necessarily and has generally 48 49 or 50 neutrons depending on the isotope. It includes the masses of the 3 subatomic particles that make up an atom. The nucleus consists of 32 protons red and 41 neutrons orange.
What isotope has 32 protons. Which pure substance can be classified as an element. There are 38 positively charged particles in its nucleus.
1 u has a value of 1660 539 066 6050 10 27 kg. The elements with the most isotopes are cesium and xenon with 36 known isotopes. Germanium - Element information properties and uses Periodic Table.
The other way is to write out the. An element has an ion with 2 chargeIt has 38 electrons and 51 neutrons. What element has 7 protons 8 neutrons and 7 electrons Questioned by administrator 27062021 in Chemistry viewed by 144 persons What is the mass number of an atom with 7 protons 8 neutrons and 7 electrons.
What atom has 32 protons and 38 neutrons. Diagram of the nuclear composition electron configuration chemical data and valence orbitals of an atom of germanium-73 atomic number. Strontium has 38 protons 50 neutrons and 38 electrons.
All elements have a number of isotopesHydrogen has the fewest number of isotopes with only three. An atom contains 36 electrons 38 protons and 50 neutrons what element or ion is being described. Physics An iron ion contains 26 protons 27 neutrons and 23 electrons.
What is the ATOMIC MASS of this element. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol ZThe total electrical charge of the nucleus is therefore Ze where e elementary charge equals to 1602 x 10-19 coulombs. Protons and Neutrons in Strontium.
For strontium Z 38. Protons neutrons and electrons. By referring to a periodic table or table of elements we see that phosphorus symbol P has an atomic number of 15.
Which substance is a mixture. For strontium Z 38. How many electrons does neon have in its first shell.
An atom contains 36 electrons 38 protons and 50 neutrons what element or ion is being described. This problem has been solved. Yttrium has 39.
Click to see full answer. What is the symbol of an element with 15 protons 16 neutrons and 18 electrons. Strontium has 38 protons necessarily and 38 electrons necessarily and has generally 48 49 or 50 neutrons depending on the isotope.
See the answer See the answer See the answer done loading. Strontium is a chemical element with atomic number 38 which means there are 38 protons in its nucleus. 4 rows What element contains 50 neutrons.
C Neon has an atomic number of 10 and an atomic mass of approximately 20. An element has 43 protons and 50 neutrons. Strontium has 38 protons necessarily and 38 electrons necessarily and has generally 48 49 or 50 neutrons depending on the isotope.

Strontium Protons Neutrons Electrons Electron Configuration
0 Response to "What Element Has 38 Protons and 50 Neutrons"
Post a Comment